In vitro plant regeneration holds great potential in the agriculture and horticulture, especially in crop improvement and hybrid breeding. Moreover, it is also applicable to studies of plant developmental regulatory mechanisms. For in vitro embryogenesis, the most important step is to re-establish the totipotency and further develop into an embryo. Embryo cell fate can be established from different tissue culture. Among the different pathways, we focus on microspore embryogenesis, where haploid embryos arise from stress-induced microspore culture.
Microspores are pollen-precursor cells in the anther, which are developmentally programmed to develop into pollen grains. Intriguingly, under certain stress conditions, microspores can be re-programmed to develop into haploid embryos. However, this process is highly inefficient or even completely absent in most crop cultivars.
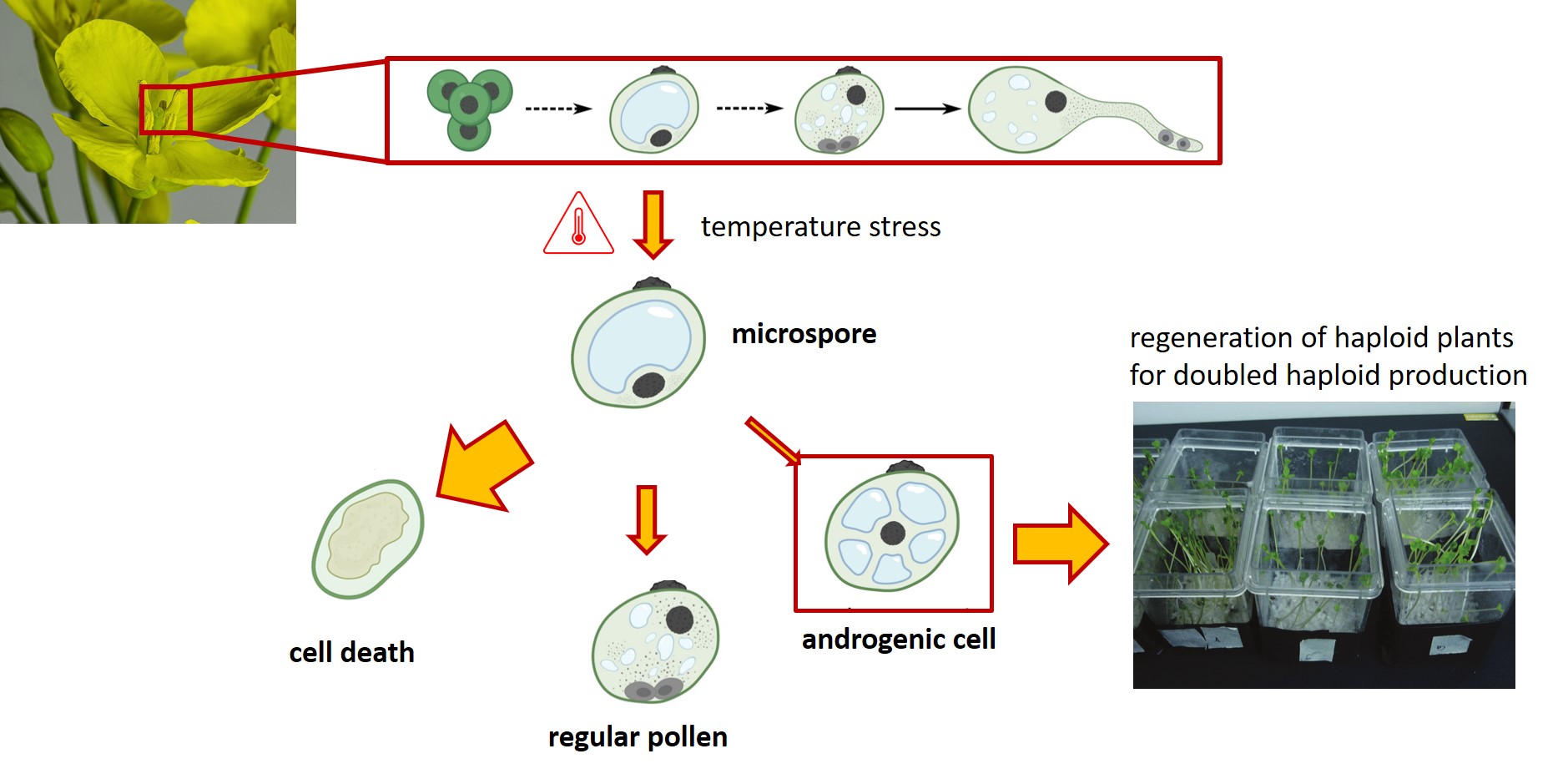
We use rapeseed (Brassica napus L.) as a model species to study microspore embryogenesis, where heat shock causes the microspores to change its developmental fate from pollen development to either developmental arrest, cell death, or embryogenesis (Corral-Martinez et al., 2020).
Given the Nowack lab's expertise and interest in Programmed Cell Death (PCD), we are focusing on understanding the cell death pathway as a major factor lowering the efficiency of microspore embryogenesis. We are investigating the cellular and molecular hallmarks associated with heat-induces microspore cell death, with the aim to identify the gene regulatory networks that promote cell death. Next, we will modulate key regulatory genes with the aim to enhance embryogenic potential by inhibiting microspore cell death.
In addition to the Nowack lab, team GENESIS consists of experts in plant regeneration and hormone signalling (Geelen lab at Ghent University) and 3 labs from the VIB-UGent Center for Plant Systems Biology: Advanced Live Cell Imaging (Vandamme lab), Oxidative Stress Signaling (Van Breusegem lab), and Inter-organelle Signalling (De Clercq lab). Together, GENESIS will provide a concerted molecular approach to understand the mechanism in a cellular level behind microspore embryogenesis. The outcome of the project will lead to a solid molecular framework of androgenesis and novel, effective strategies for boosting the efficiency of stress-induced microspore embryogenesis and its wider application in hybrid breeding.